a) National Bureau of Plant Genetic Resources (NBPGR)
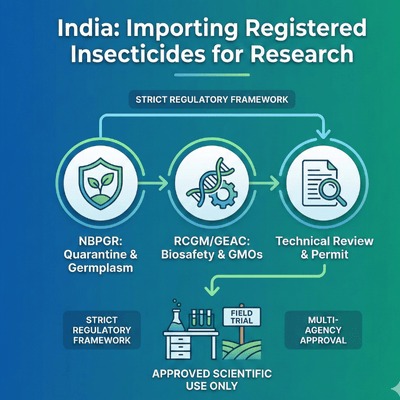
NBPGR is the central authority for importing plant germplasm and research materials. It screens applications, verifies research objectives, evaluates quarantine risks, ensures non-commercial use, and coordinates inspection and post-entry monitoring.
b) Review Committee on Genetic Manipulation (RCGM) – DBT
RCGM oversees all GMO-related research. It reviews genetic manipulation data, laboratory containment levels, biosafety protocols, and assesses potential environmental risks before granting research approvals.
c) Genetic Engineering Approval Committee (GEAC)
GEAC, under the Environment (Protection) Act, 1986, evaluates genetically engineered organisms/products, approves their import, export, field trials, or controlled release, and enforces biosafety and environmental safeguards.
d) ICAR, SAUs & International Research Centres
These research institutions act as applicants/collaborators in trials involving insecticides or biological materials. Their internal Institutional Biosafety Committees (IBCs) participate in reviewing, endorsing, and forwarding applications for regulatory approvals.
This approval applies to the import of items listed under Schedule-V, Schedule-VI, and Schedule-XII of the Plant Quarantine (Regulation of Import into India) Order, 2003.
It is required when the import is intended for non-commercial scientific use, including:
Note: This approval does not apply to commercial-scale insecticide imports meant for sale or distribution. Such cases require separate processing under GEAC guidelines and registration under the Insecticides Act, 1968.
The following categories of applicants are eligible to seek approval for the import of materials covered under Schedule-V, Schedule-VI, and Schedule-XII of the Plant Quarantine (Regulation of Import into India) Order, 2003:
a) Importers
Any individual or company holding valid business registration and authorized to engage in the import of agrochemicals, biological materials, germplasm, transgenics, or related research inputs. The applicant must possess the necessary statutory licences required under applicable Indian laws.
b) Authorised Agents
Legally appointed representatives or agents who have been formally empowered by an importer to act on their behalf. Such agents submit applications, coordinate documentation, and comply with regulatory requirements as per the authorization issued to them.
c) Research Institutions
Eligible research-based entities involved in agricultural, biological, or environmental studies, including:
These institutions typically import research materials for experimentation, field evaluation, breeding programmes, or laboratory analysis.
d) International Scientific Organisations
Recognized global research bodies engaged in collaborative programmes with Indian agencies, such as:
These organisations are permitted to import germplasm, biological agents, or experimental material for joint research initiatives with Indian partners.
A. Technical Information
B. Safety Documents
C. Research Proposal
D. Containment & Quarantine Compliance
E. Legal & Institutional Approvals
F. Shipping & Logistics Information
Step 1: Prepare the Application Dossier
Compile all mandatory technical, safety, research, and quarantine documents. Only complete dossiers are accepted.
Step 2: Submit Application to the Director, NBPGR
Submit as per the current NBPGR format, including:
Step 3: NBPGR Preliminary Scrutiny
NBPGR checks completeness, purpose of import, schedule category, and quarantine readiness, Clarifications requested.
Step 4: Technical Review
Authorities ask for additional information or verify research facility preparedness.
Step 5: Permit Issuance
NBPGR issues the import permit with conditions on quantity, validity, research site, and mandatory quarantine/safety requirements.
Step 6: Port-of-Entry Verification
Plant Quarantine officials check documents and inspect the consignment on arrival.
Step 7: Post-Import Compliance
Importer must use the material strictly for approved research, maintain records, follow disposal norms, and submit reports.
Importing an insecticide into India—whether or not it is registered abroad—requires strict compliance with national biosafety and plant quarantine regulations. The process is intentionally rigorous to protect India’s agriculture, prevent pest or pathogen entry, and ensure that research materials are used responsibly.
NBPGR oversees the approval pathway, allowing imports only when the scientific purpose is justified and supported by complete technical documentation, appropriate biosafety clearances (such as RCGM/GEAC for GM materials), and adequate quarantine arrangements. Each stage of review—from dossier examination to facility verification and port-of-entry inspection—ensures that all phytosanitary and environmental risks are carefully evaluated.
For more details, please visit Metacorp





We are the pioneers in offering environmental consulting services to our patrons, giving us the first mover advantage & keeping us ahead of our competitors.
Very experienced in filing, monitoring & submission of CDSCO Compliances, Drugs Manufacturing & sale guidelines, Environmental Impact Assessment, AERB consulting services, Pollution Control Board CTE & CTO Advisory Services, Waste Management Authorization from State Pollution Control Boards, Fertilizers & Insecticides Manufacturing, Wholesale & Import Compliances
