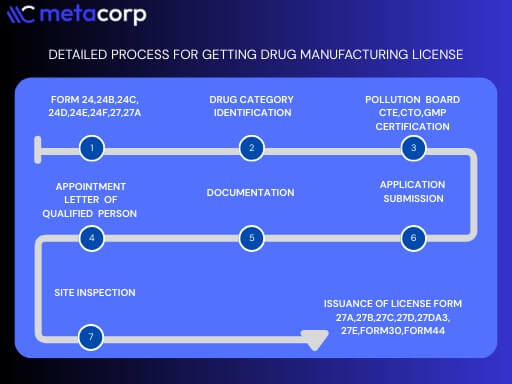
To manufacture drugs legally in India, it is mandatory to obtain a manufacturing license under the Drugs and Cosmetics Act, 1940. The process is governed by both State and Central Regulatory Authorities, depending on the nature and category of the drugs.
The first step is to determine the type of manufacturing license applicable to your business. This could include licenses for Allopathic Drugs, Ayurvedic, Unani, Siddha, Homeopathic Medicines, Cosmetics, or Medical Devices. Additionally, if the company does not own a manufacturing unit but utilizes another facility, a Loan License may be required.
The applicant must set up a manufacturing unit that complies with Good Manufacturing Practices (GMP) as outlined in Schedule M of the Drugs and Cosmetics Rules. The infrastructure should have separate areas for manufacturing, packaging, storage, and quality control, with proper ventilation, hygiene, and waste disposal systems. The premises must also include cold storage and equipment suitable for the type of drugs being produced.
A critical requirement for obtaining the license is the appointment of qualified personnel. This includes a Manufacturing Chemist, who should have a degree in Pharmacy or Pharmaceutical Sciences and relevant industry experience, and an Analytical Chemist, who is responsible for quality control and testing of the products. The qualifications and experience must be documented and submitted with the application.
The applicant must prepare a comprehensive set of documents, which includes:
The completed application along with the necessary documents must be submitted to the State Drugs Standard Control Organization (SDSCO) or, in specific cases involving critical drugs or new drug molecules, to the Central Drugs Standard Control Organization (CDSCO). Applications are made in specific forms, such as Form 24 for allopathic drugs or Form 27 for Ayurvedic products.
After submission, the drug inspector from the licensing authority conducts a thorough inspection of the manufacturing premises. The inspection evaluates the infrastructure, hygiene standards, personnel qualifications, and compliance with Schedule M. Any deficiencies must be rectified before the license is approved.
Upon successful inspection and verification of all submitted documents, the drugs manufacturing license is granted in the prescribed format (e.g., Form 25 for allopathic drugs, Form 28 for other categories). The license remains valid for a period of five years and must be renewed periodically to ensure continued compliance with regulatory requirements.
Once the license is issued, the license holder must ensure ongoing compliance with all regulatory norms. This includes maintaining batch manufacturing records, conducting regular in-house quality checks, submitting periodic returns to the authority, and being prepared for surprise inspections. Any violations or deviations may result in suspension or cancellation of the license.
Form 24: Application to manufacture drugs (except those in Schedules C, C (1), and X).
Form 24B: Application for license to repack drugs for sale (excluding Schedules C, C (1), and X).
Form 24C: Application to manufacture and sell homoeopathic medicines or potentised preparations.
Form 24D: Application to manufacture Ayurvedic, Siddha, or Unani drugs.
Form 24E: Loan license application to manufacture Ayurvedic/Siddha/Unani drugs.
Form 24F: Application to manufacture drugs listed in Schedule X (excluding Schedules C & C (1)).
Form 27: Application to manufacture drugs under Schedules C & C (1) (excluding part XB and Schedule X).
Form 27A: Loan license application for drugs under Schedules C & C (1) (excluding part XB and Schedule X).
Form 27B: License to manufacture drugs listed under Schedules C, C (1), and X.
Form 27C: License for operating a Blood Bank for blood processing and component preparation.
Form 27D: License to manufacture Large Volume Parenterals, Sera, Vaccines, or r-DNA derived drugs (excluding Schedule X).
Form 27DA3: Loan license to manufacture Large Volume Parenterals, Sera, Vaccines, or r-DNA derived drugs (excluding Schedule X).
Form 27E: License to manufacture blood products for sale or distribution.
Form 30: License to manufacture drugs for examination, test, or analysis purposes.
Form 44: Permission to import/manufacture new drugs or conduct clinical trials.
For more details, please click Drugs Licensing





We are the pioneers in offering environmental consulting services to our patrons, giving us the first mover advantage & keeping us ahead of our competitors.
Very experienced in filing, monitoring & submission of CDSCO Compliances, Drugs Manufacturing & sale guidelines, Environmental Impact Assessment, AERB consulting services, Pollution Control Board CTE & CTO Advisory Services, Waste Management Authorization from State Pollution Control Boards, Fertilizers & Insecticides Manufacturing, Wholesale & Import Compliances
