Introduction
- A Free Sale Certificate (FSC) is a critical regulatory document for manufacturers and exporters seeking to expand their business in international markets. It acts as an official endorsement that a product—be it a medical device, pharmaceutical, cosmetic, or biological material—is legally manufactured, marketed, and sold in the country of origin. Essentially, an FSC assures foreign authorities and buyers that the product meets national safety, quality, and regulatory standards, making it eligible for import without restrictions.
- In India, the Central Drugs Standard Control Organization (CDSCO) is the designated authority responsible for issuing FSCs for medical devices and related products. Obtaining an FSC from CDSCO not only validates the compliance and legitimacy of your products but also facilitates smoother regulatory approvals in overseas markets. For exporters, it serves as a confirmation to product quality, building trust with international buyers and enhancing the brand’s credibility.
- Whether you are a startup entering global markets or an established manufacturer looking to expand, securing a Free Sale Certificate is a fundamental step in ensuring your products are recognized and accepted worldwide.
What is a Free Sale Certificate (FSC)?
A Free Sale Certificate (FSC) is an official document issued by the competent authority in India, confirming that a product is legally manufactured, marketed, and sold in the country. It serves as an assurance to foreign regulatory authorities and international buyers that the product meets Indian regulatory requirements and adheres to safety and quality standards.
The FSC certifies that:
- The product is legally manufactured and sold in India.
- The product complies with all applicable Indian regulations.
- The product is safe for use and meets established quality standards.
In essence, the FSC provides validation of the product’s legitimacy, safety, and quality, making the export process smoother and enhancing trust with overseas buyers.
Products Covered under Free Sale Certificate
A Free Sale Certificate (FSC) is primarily issued for products that require international recognition of their legal manufacture, safety, and quality. The FSC ensures that foreign regulatory authorities and global buyers can trust the product’s compliance with Indian regulations. The key product categories typically covered include:
- Medical Devices: This includes diagnostics, surgical instruments, implants, and other regulated medical equipment. FSCs for medical devices validate that these products meet stringent quality and safety standards, which is crucial for international distribution.
- Food Products: Packaged foods, dietary supplements, nutraceuticals, and health-focused consumables fall under this category. The FSC confirms that these products are manufactured in compliance with Indian food safety and quality regulations, facilitating seamless entry into foreign markets.
- Cosmetics: Skincare, haircare, personal hygiene products, and beauty formulations are included. The FSC serves as proof that these products are safe, legally marketed in India, and adhere to regulatory quality norms.
- Biological Products: Vaccines, sera, enzymes, and other biological or biotechnological materials require FSCs to assure international authorities of their safety, efficacy, and compliance with Indian regulations.
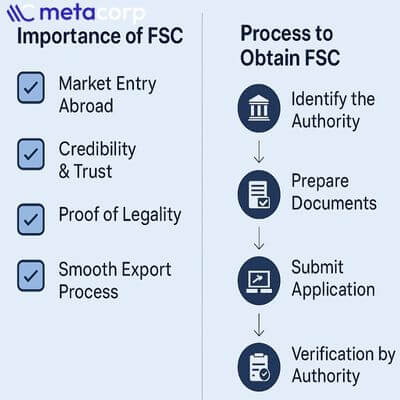
Why a Free Sale Certificate (FSC) is Important
A Free Sale Certificate (FSC) plays a crucial role for Indian manufacturers and exporters seeking to reach global markets. Its importance extends beyond regulatory compliance to building trust and facilitating business expansion:
- Proof of Legal Compliance: An FSC confirms that the product is manufactured, marketed, and freely sold in India according to national safety and quality standards.
- Streamlines International Trade: Many countries require an FSC for import clearance. Possessing one helps reduce delays in customs and regulatory approvals, ensuring smoother cross-border shipments.
- Enhances Credibility: The certificate demonstrates that the product has undergone regulatory scrutiny, instilling confidence among international buyers, distributors, and authorities.
- Supports Market Entry: For businesses entering new markets, an FSC provides reassurance that the product adheres to recognized standards, making it easier to gain acceptance and compete effectively.
- Complements Other Approvals: While it does not replace country-specific registrations or permits, an FSC serves as formal evidence of product legitimacy, safety, and quality, often forming a prerequisite for additional certifications abroad.
Procedure to Obtain FSC from CDSCO
Obtaining a Free Sale Certificate (FSC) from CDSCO ensures your products comply with Indian regulatory standards. The process includes:
1. Application Submission
Submit an online application with required documents:
- Product details (composition, specifications, intended use)
- Manufacturing license or registration certificate
- Existing regulatory approvals
- Quality certifications (ISO, GMP, etc.)
- Declarations or FSC requests from other authorities
2. Document Verification
CDSCO reviews the application and documents to confirm compliance with Indian regulations and safety standards.
3. Quality Inspection
Certain products undergo factory inspections to verify adherence to Good Manufacturing Practices (GMP).
4. FSC Issuance
once verified, CDSCO issues the FSC, including product details, manufacturer information, and confirmation that the product is legally manufactured and sold in India.
Receiving an FSC validates compliance, enhances credibility, and supports seamless international trade.
Key Points to Consider About Free Sale Certificates (FSC)
- Validity Period: A Free Sale Certificate is generally valid for a limited duration, typically one year from the date of issuance. Exporters should monitor the expiry and ensure timely renewal to maintain uninterrupted international trade.
- Country-Specific Requirements: Different countries have unique regulatory requirements for FSCs. Exporters must verify the importing country’s guidelines to ensure the certificate is accepted and meets all necessary standards.
- Complementary Approvals: An FSC does not replace other mandatory approvals, such as product registration, import permits, or certifications required by the destination country. Exporters must obtain all additional clearances to comply fully with foreign regulations.
Conclusion
A Free Sale Certificate (FSC) is more than just a regulatory document—it is a vital tool for Indian manufacturers and exporters aiming to establish a strong presence in global markets. By certifying that a product is legally manufactured, safe, and compliant with Indian regulations, an FSC not only facilitates smoother international trade but also enhances credibility and trust with overseas buyers and regulatory authorities.
Whether you are exporting medical devices, food products, cosmetics, or biological materials, securing an FSC demonstrates your commitment to quality, safety, and regulatory compliance. Staying aware of country-specific requirements, validity periods, and complementary approvals ensures that your products are always ready for global distribution.
For more details, please visit Metacorp