India is globally recognized as a pharmaceutical powerhouse—ranking among the top in the production of generic drugs, vaccines, and bulk Active Pharmaceutical Ingredients (APIs). With a well-established scientific infrastructure and cost-effective manufacturing capabilities, the country attracts both domestic and international players seeking to enter the pharmaceutical sector.
However, establishing a drug manufacturing unit in India is not just about infrastructure and investment—it involves navigating a robust regulatory framework designed to ensure the safety, efficacy, and quality of pharmaceutical products. Missing even a single legal step can lead to operational delays, regulatory penalties, or even license cancellation.
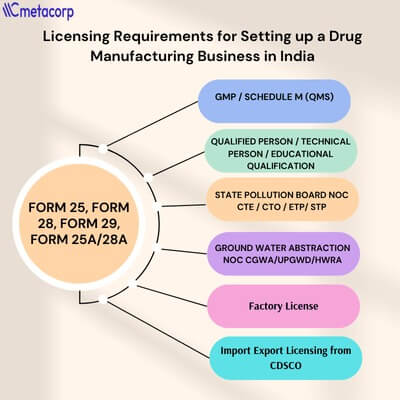
This comprehensive guide outlines the essential licensing and compliance requirements for setting up a drug manufacturing business in India, helping you stay legally compliant and audit-ready from day one.
This license is the foundation of your pharmaceutical business. It is issued by the State Drugs Control Authority under the Drugs and Cosmetics Act, 1940. It legally permits the manufacturing of pharmaceutical products for sale and distribution.
Types of Manufacturing Licenses:
Form 25 – License to manufacture allopathic drugs not specified in Schedule C, C1.
Form 28 – License to manufacture drugs specified in Schedule C and C1 (e.g., vaccines, injectables).
Form 29 – License to manufacture drugs for the purpose of examination, test, or analysis.
Loan License (Form 25A/28A) – For units operating using another manufacturer's infrastructure.
Requirements:
A drug manufacturing business must employ at least one competent technical person to supervise manufacturing operations.
Eligibility Criteria:
Significance:
Qualified personnel are crucial for ensuring adherence to regulatory guidelines, maintaining product quality, and avoiding non-compliance penalties. Their expertise directly impacts the efficiency and credibility of manufacturing operations.
Regulatory Requirement:
Manufacturing units are required to obtain environmental clearances from the State Pollution Control Board (SPCB) under the Air and Water (Prevention and Control of Pollution) Acts.
Types of Consent:
Environmental Compliance Includes:
Importance:
Environmental compliance is non-negotiable. Failure to comply can lead to license suspension, plant closure, or legal penalties.
If your manufacturing unit uses groundwater for operations, you must obtain a NOC from the Central Ground Water Authority (CGWA) or State Ground Water Board.
Role in Operations:
Sustainable water sourcing is critical to avoid depletion and legal complications. This NOC ensures that your unit operates responsibly within groundwater extraction limits.
Process:
Role: Ensures responsible water usage, supports sustainability goals, and prevents legal or environmental conflicts.
This is a mandatory license issued by the Department of Labour or Chief Inspector of Factories for premises employing 10 or more workers with power, or 20+ without power.
Key Requirements:
Compliance Relevance:
This license ensures that the working environment is safe, legally compliant, and geared toward worker welfare—thereby reducing liability and increasing operational efficiency.
A No Objection Certificate (NOC) from the local Fire Department certifies that your premises follow fire safety regulations.
Requirements:
Operational Importance:
A Fire NOC not only safeguards life and property but is also mandatory for other government clearances, insurance policies, and licensing continuity.
In the pharmaceutical industry, where public health and safety are paramount, the implementation of a robust Quality Management System (QMS) and adherence to Good Manufacturing Practices (GMP) are not just regulatory requirements—they are fundamental to business integrity and product credibility.
A well-established QMS ensures that drugs are manufactured consistently to the highest standards of quality, safety, and efficacy, in compliance with both Indian and international regulatory frameworks. It forms the backbone of a company’s ability to maintain regulatory approvals, pass inspections, and expand into regulated markets.
Key Components:
Strategic Value:
A robust QMS framework enhances brand credibility, supports regulatory audits, and enables smoother entry into global markets—making it a critical pillar of pharmaceutical operations.
In the pharmaceutical industry, the supply chain often extends beyond national borders—whether it’s importing raw materials such as Active Pharmaceutical Ingredients (APIs), excipients, or packaging materials, or exporting finished drug formulations to global markets. These activities are strictly regulated to ensure the safety, quality, and traceability of pharmaceutical products.
Engaging in import and export operations requires multiple licenses and registrations under Indian and international regulatory frameworks.
Must-Have Registrations:
International Certifications:
For exporting to regulated markets, you may also need:
Global Significance:
These approvals unlock access to international markets, boost competitiveness, and demonstrate adherence to global pharmaceutical standards.
Setting up a drug manufacturing unit in India is a high-stakes venture that goes far beyond building infrastructure or acquiring capital. It demands meticulous planning, regulatory foresight, and unwavering commitment to compliance at every stage of the business lifecycle. From securing essential licenses like the Drug Manufacturing License and Factory License to adhering to environmental regulations, QMS protocols, and international export standards—each step is critical to establishing a legally compliant, operationally efficient, and globally competitive pharmaceutical enterprise.
By proactively meeting these regulatory requirements, businesses not only mitigate legal and operational risks but also position themselves for long-term growth, global market access, and brand trust in an industry where quality and compliance are non-negotiable.
For more details, please visit Metacorp Pharma Licensing





We are the pioneers in offering environmental consulting services to our patrons, giving us the first mover advantage & keeping us ahead of our competitors.
Very experienced in filing, monitoring & submission of CDSCO Compliances, Drugs Manufacturing & sale guidelines, Environmental Impact Assessment, AERB consulting services, Pollution Control Board CTE & CTO Advisory Services, Waste Management Authorization from State Pollution Control Boards, Fertilizers & Insecticides Manufacturing, Wholesale & Import Compliances
