The Indian Pharmaceutical Business revenue over the last two decades has grown rapidly. The country has made a name for itself as one of the largest global centers for research and production. The vast majority of medicines sold in India are manufactured there and are in high demand.
As a consequence of the high demand for pharma products, the foreign trade of pharmaceutical products experienced a substantial boom. Starting a Pharmaceutical business in India complying with several regulations and obtaining compulsory licenses and permits. Multifarious Documents Required To Start Pharmaceutical Medicine Export Business in India, here is a general list of what you may need to start a pharma business in the industry.
Business registration is a foundational aspect of an entity looking to operate legally and establish itself as a legitimate pharma export business. The importance of business registration spans several aspects of credibility, financial transactions, legal compliance, and overall business operations.
It is a legal document that is issued by the regulatory authorities. It is vital for the pharmaceutical industry that offer complete public safety, quality assurance, regulatory oversights, traceability, control of drug distribution, and International trade freedom.
GST is one of the necessary Documents Required To Start a Pharmaceutical Medicine Export Business in India, including other countries.GST is like a comprehensive indirect tax that has replaced several indirect taxes.
In the pharma business, you never negotiate the quality of products. Quality management certifications are commitment to quality, and building trust among customers, partners, and stakeholders. It is an internationally recognized standard that an organization can achieve to demonstrate commitment.
Pharmaceutical Registration is a complicated procedure that needs collaboration between pharmaceutical companies and regulatory authorities to ensure that patients have safe and effective medicines.
Transportation and logistics include the movement of pharma products from one location to another. Several documents are used to facilitate and manage this procedure that ensures that pharma product shipments are accurately documented.
The export Contract agreement is one of the Documents Required To Start a Pharmaceutical Medicine Export Business in India. It is a legal contract specifying terms and conditions between the exporter and importer in the context of International trade.
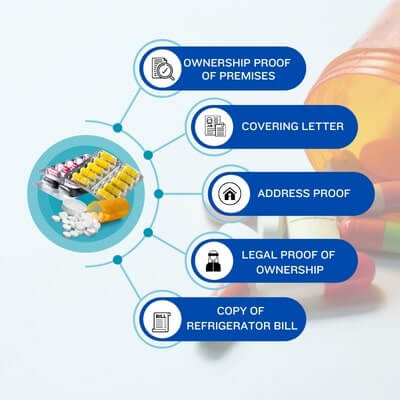
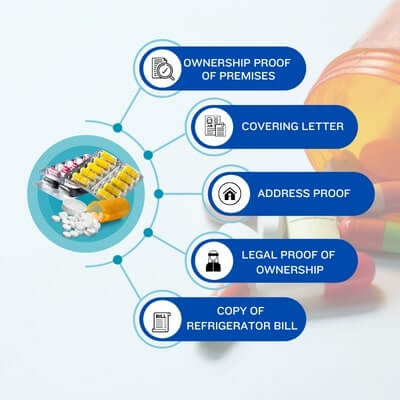
The export of pharmaceutical products requires a lot of licensing and certificates from the different authorities in India. Below we mentioned some Documents Required To Start a Pharmaceutical Medicine Export Business in India:
Pharma is a very vast area and joining the pharma industry offers you high-margin profit with high returns. It provides multifarious advantages:
Mostly we see pharma experts confused about how to export their products, to help you out we mentioned some important tips to run your pharma business smoothly in the international level:
For more details, please visit Metacorp





We are the pioneers in offering environmental consulting services to our patrons, giving us the first mover advantage & keeping us ahead of our competitors.
Very experienced in filing, monitoring & submission of CDSCO Compliances, Drugs Manufacturing & sale guidelines, Environmental Impact Assessment, AERB consulting services, Pollution Control Board CTE & CTO Advisory Services, Waste Management Authorization from State Pollution Control Boards, Fertilizers & Insecticides Manufacturing, Wholesale & Import Compliances
